Abcam's Pyruvate Kinase (PK) Assay Kit provides a simple, direct and automationready procedure for measuring pyruvate kinase activity in cell culture supernatant, plasma, serum, tissue, urine and other biological fluids (not validated for use with whole blood) In the assay, PEP and ADP were catalyzed by PK to generate pyruvate and ATP The generated pyruvate is oxidized by pyruvatePK Assay By identifying and understanding the pharmacokinetics of a given drug or medicine, scientists and researchers get to know its essential propertiesHow effective the drug is, what is the required dosage in which this should be administered are some of the things which can be brought out with this investigative procedure known as PK AssayAntiID Antibodies and PK Assays Because most antiID antibodies are generated against a specific antibody drug, they are normally used in preclinical practices for PK analysis PK is the study of drug metabolism throughout the body
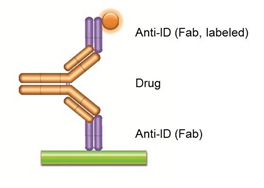
Effective Tools For Drug Monitoring Assays Bio Rad
Pk assay fda guidance
Pk assay fda guidance-The generic nature of the PK assay was achieved by using epitopes present on all relevant IgG subclasses (IgG1, IgG2, IgG4) for assay design The assay was also designed to generate signal from human IgG only Immune reagents were therefore selected that would not crossreact with IgG in species employed for in vivo experiments (primarily PK assay bioanalytical testing methods are used to determine concentration time profiles of the drug and metabolites in biological sample fluids, providing information necessary for PK analysis PK assays are a vital component of the drug development process, and the data derived is used to help select dosage for preclinical and clinical studies
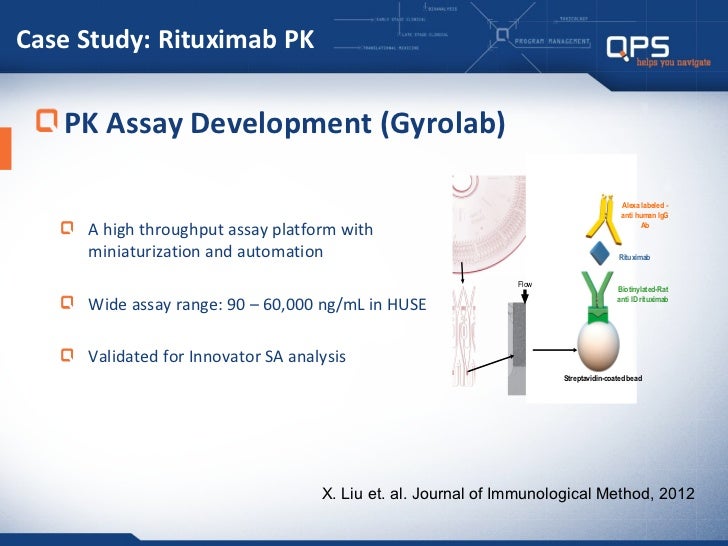



Qps Biosimilar Bioanalytical Approaches
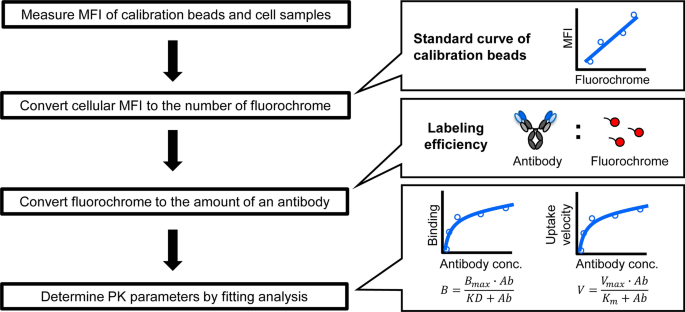
To interpret pharmacokinetic (PK) data of biotherapeutics, it is critical to understand which drug species is being measured by the PK assay For therapeutic antibodies, it is generally accepted that "free" circulating antibodies are the pharmacologically active form needed to determine the PK/pharmacodynamic (PD) relationship, safety margin calculations, and dose projections fromPharmacokinetic (PK) Assays Precision advances the development of complex therapeutics including small peptides, antibodydrug conjugates (ADCs), PEGylated proteins, monoclonal and bispecific antibodies, and cellbased therapies like CART, with a range of bioanlytical servicesThe Pyruvate Kinase Activity Assay Kit provides a simple and direct procedure for measuring pyruvate kinase activity in a variety of biological samples Pyruvate concentration is determined by a coupled enzyme assay, which results in a colorimetric (570 nm)/ fluorometric (λex = 535/λem = 587 nm) product, proportional to the pyruvate present
PK Assay and PK Analysis Services by NorthEast BioLab We offer a wide range of Pharmacokinetic (PK) Assays beginning from the early discovery phase when a potential drug compound is administered to rodents for understanding ADME propertiesPharmacokinetics (from Ancient Greek pharmakon "drug" and kinetikos "moving, putting in motion"; ROLE SUMMARY As a member of the Clinical Assay Group within Global Clinical Pharmacology, Clinical Assay Lead will play a critical role in supporting clinical strategies in all stages of drug development and post marketing activities through scientific and technical oversight and management of external and internal partners involved in delivering quality, timely, and regulatory compliant PK
A typical suite of assays required during biotherapeutic development includes drug concentration assay (pharmacokinetic or PK assay) and assays detecting presence of antidrug immune response Multiple assays may be required to determine appropriate compound PK as well as to characterize an antidrug immune responsePharmacokinetics (PK) studies are of great importance in creating new therapeutic drugs, understanding both the beneficial and harmful effects of drugs, and guiding drug development experiments and trials In PK assays, the applied assay method should be well characterized and fully validated, in order to yield reliable results Pyruvate Kinase Assay Kit ab432 provides a simple, direct and automationready assay for measuring pyruvate kinase activity in cell culture supernatant, plasma, serum, tissue, urine and other biological fluids (not validated for use with whole blood)
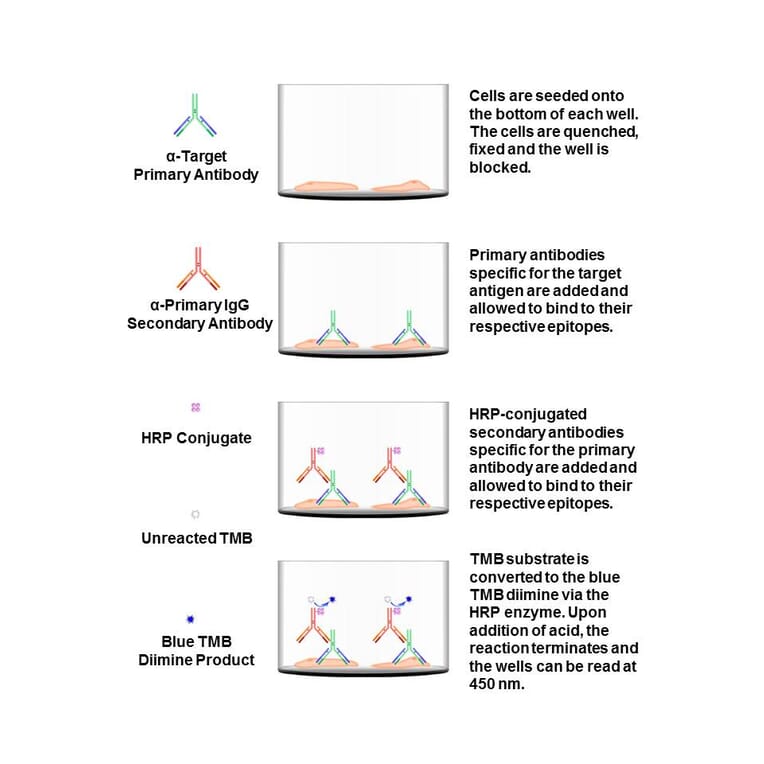



Dna Pk Cell Based Elisa Kit A Antibodies Com




Anti Peg Monoclonal Igm Antibody Anpeg 1 Anp Technologies
See chemical kinetics), sometimes abbreviated as PK, is a branch of pharmacology dedicated to determine the fate of substances administered to a living organismOr ADA on the PK assay need to be considered in the design of the sampling strategy by looking for active or total drug Chirmule et al, AAPS J 14(2), (12) Ø The success of the PK–PD modeling effort depends on close communication between the PK–PD bioanalytical and clinical scientists The PK–PD model isSensitive and reproducible pharmacokinetic (PK) and immune response (IR) assays are required as part of the complex and lengthy development process for biotherapeutic proteins These assays are included in a range of approaches applied to understand the nature and properties of the drug as well as the induction of antidrug antibodies (ADA), which can cause adverse events and loss




Pk Assay For Antibody Quantification In Sodium Heparin Plasma




Elisa Assay Kits For Therapeutic Antibodies Acrobiosystems
PK assays are a vital part of the drug development process and the data derived is used to help select dosage for clinical studies We conduct our highquality PK services strictly in compliance with GCP regulations and in accordance with international regulatory guidelines (FDA, EMEA, ICH)PK/PD/ADA Antibodies and Assays Because all biological therapeutics can induce an immune response that ranges from benign to severe adverse effects, evaluating unwanted immunogenicity is a critical step in earlystage researchrequiring both a wellconceived strategy and fitforpurpose assays for antibody detection and characterizationResults A PK assay strategy was developed that included comprehensive novel reagent and assay characterization approaches for the ADC adotrastuzumab emtansine (TDM1)




Figure 10 From Development Of Pharmacokinetic Pk Assays For Detecting Biosimilars Targeting Tnfa Using Alphalisa Semantic Scholar



E B F Eu Wp Content Uploads 18 06 Td1706 01 Surinder Kaur Pdf
Typical PK assays in ½ Area 96well plate B) AlphaPlex assay Protocol for PK assay in 384well OptiPlate A B Figure 4 Adalimumab antibody comparison A) A very sharp Hook can be seen using the high affinity (K D =006 nM) antiAdalimumab antibody (HC04) on both sides of the




Pk Pd Modeling Of Protein Drugs Implications In Assay Development Bioanalysis



2
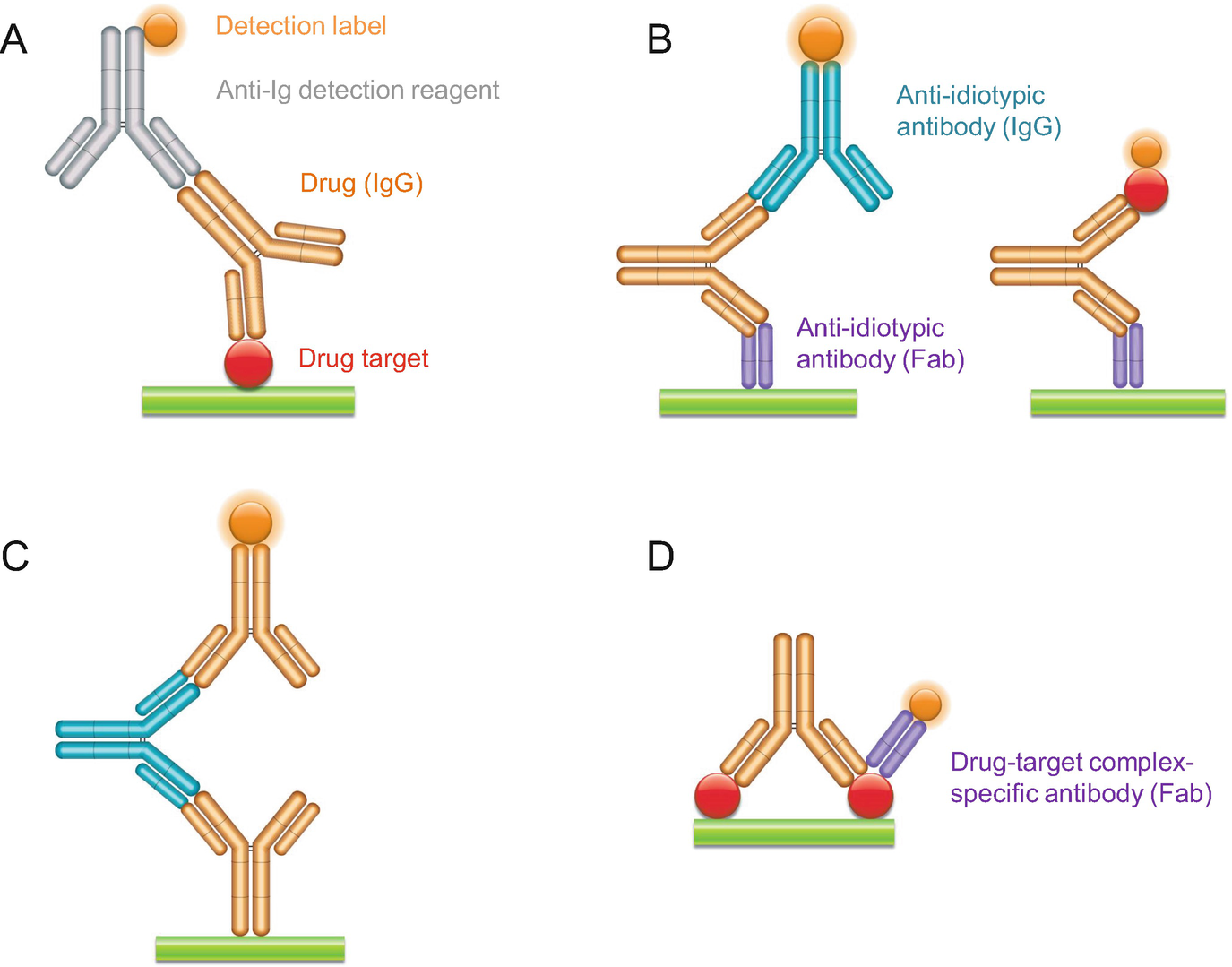



Recombinant Anti Idiotypic Antibodies In Ligand Binding Assays For Antibody Drug Development Springerlink




Pk Assay Services Eurofins Scientific




Pharmacochaperone Assays Discoverx




Assay Antibodies Crb Discovery




Qps Biosimilar Bioanalytical Approaches








Anti Peg Monoclonal Igm Antibody Anpeg 1 Anp Technologies




Ait Bioscience Introduces Expedited Discovery Pk Services Ait Bioscience




Pyruvate Kinase Assay Kit Ab432 Abcam



Establish Drug Pk Analysis Method Based On Competitive Elisa




What Is An Anti Idiotypic Antibody
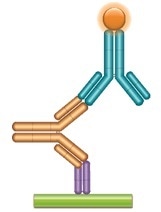



Working With Pharmacokinetic Bridging Assays
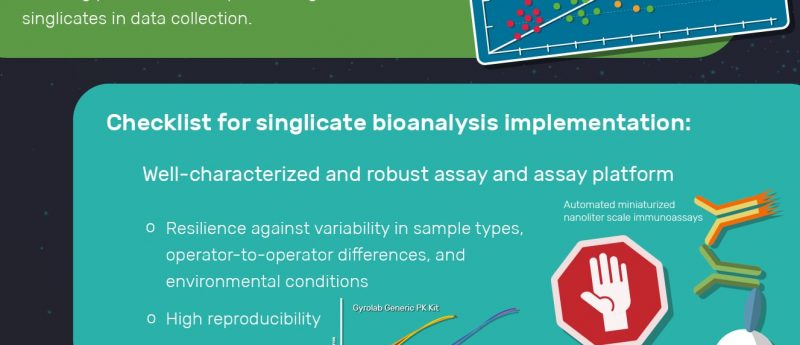



Is Your Biotherapeutic Pk Assay Singlicate Ready Bioanalysis Zone
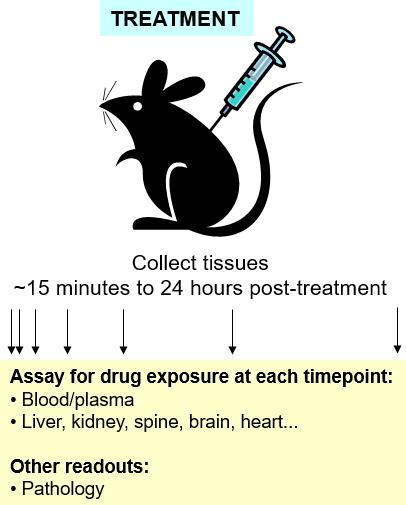



Hooke Contract Research Pharmacokinetics Pk



Ncbiologics Com Bioassays



Kinasera Com Kinasera Trf Biochemical Assay
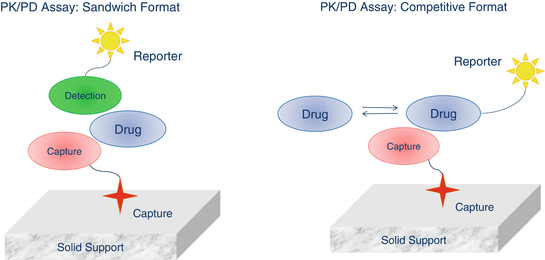



Clinical Assays For Biological Macromolecules Basicmedical Key




Biochain Institute Incmalachite Green Phosphate Assay Kit 2 500 Assays Pk Fisher Scientific
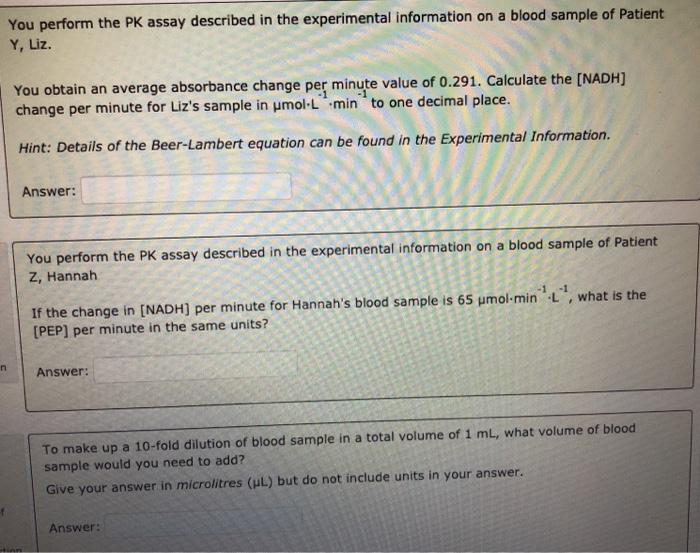



Solved You Perform The Pk Assay Described In The Experime Chegg Com




Pk And Ada Assay Development Services




Poster Presentations Release Biologics And Biosimilars
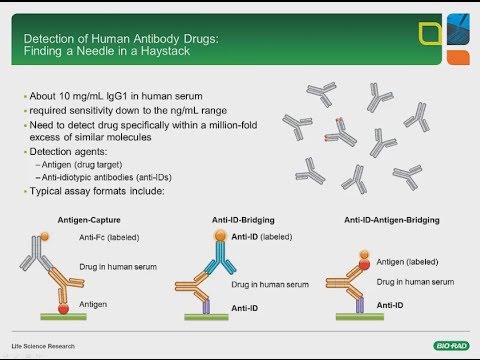



Developing Recombinant Anti Idiotypic Antibodies For Pk Pd And Immunogenicity Assays Youtube




Smc Immunogenicity Testing Assay Life Science Research Merck




Antibiotic Assay Discs 6mm 1000 Pk Lifegene
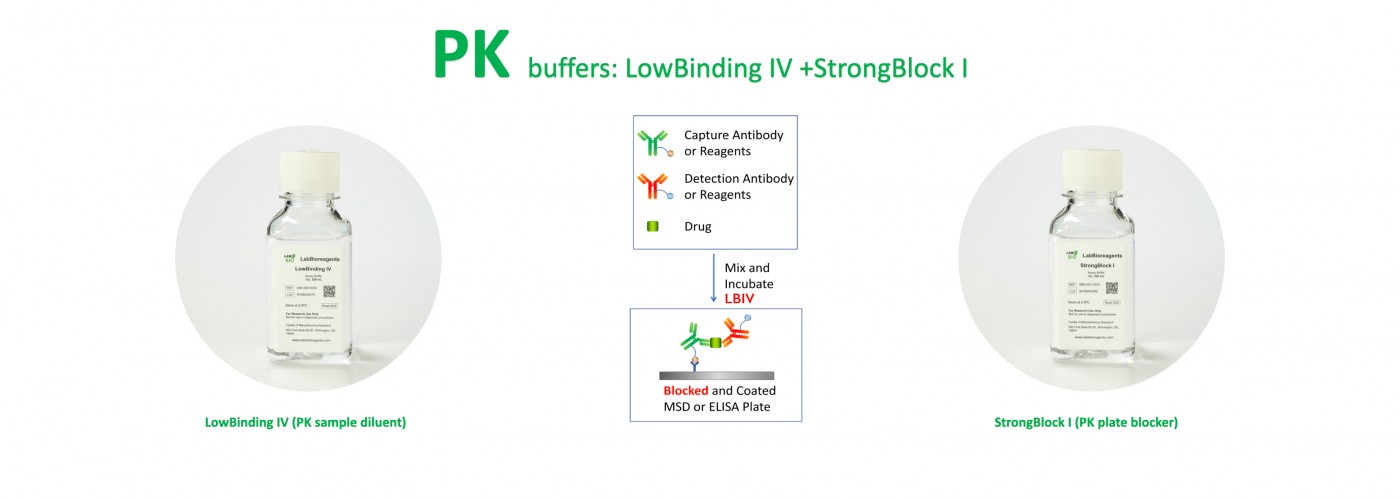



Assay Buffer




Matthias Hofmann Novartis Institutes For Biomedical Research Nibr Ppt Video Online Download
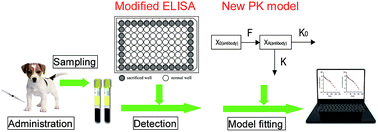



An Enzyme Linked Immunosorbent Assay With A New Way To Control The Edge Effect And Its Application For Bevacizumab Pharmacokinetic Studies In Beagle Dogs By Fitting With A New Pharmacokinetic Model Analytical
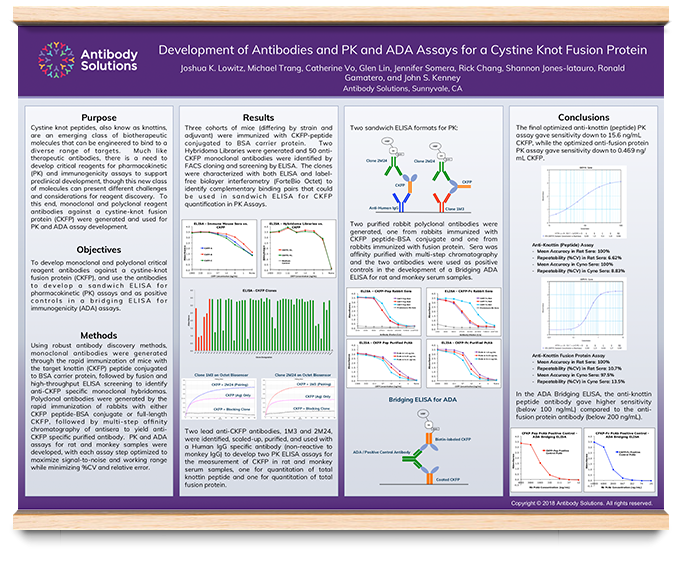



Development Of Antibody And Pk And Ada Assays For A Cystine Knot Fusion Protein
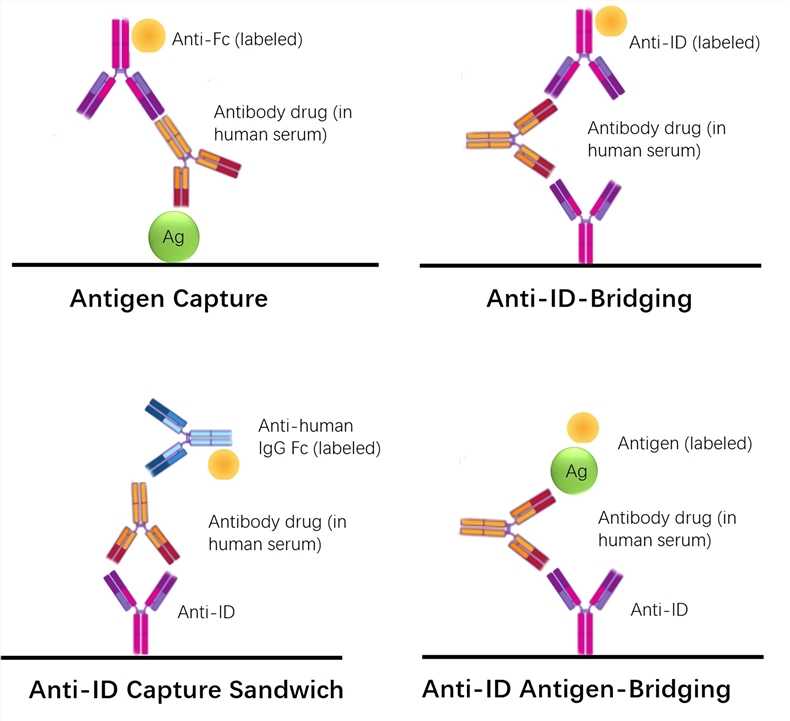



Pharmacokinetic Pk Bridging Elisa Measuring Free Drug Creative Biolabs
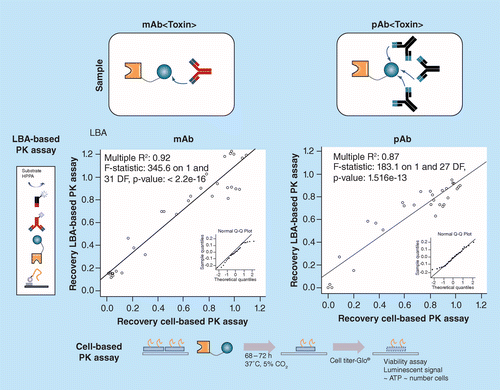



Quantification Of A Bifunctional Drug In The Presence Of An Immune Response A Ligand Binding Assay Specific For Active Drug Bioanalysis




Pk Assay Strategies For Biotherapeutics Iir




Theoretical Considerations And Practical Approaches To Address The Effect Of Anti Drug Antibody Ada On Quantification Of Biotherapeutics In Circulation Abstract Europe Pmc




Www Pharmacologicalsciences Us Drug Discovery 3
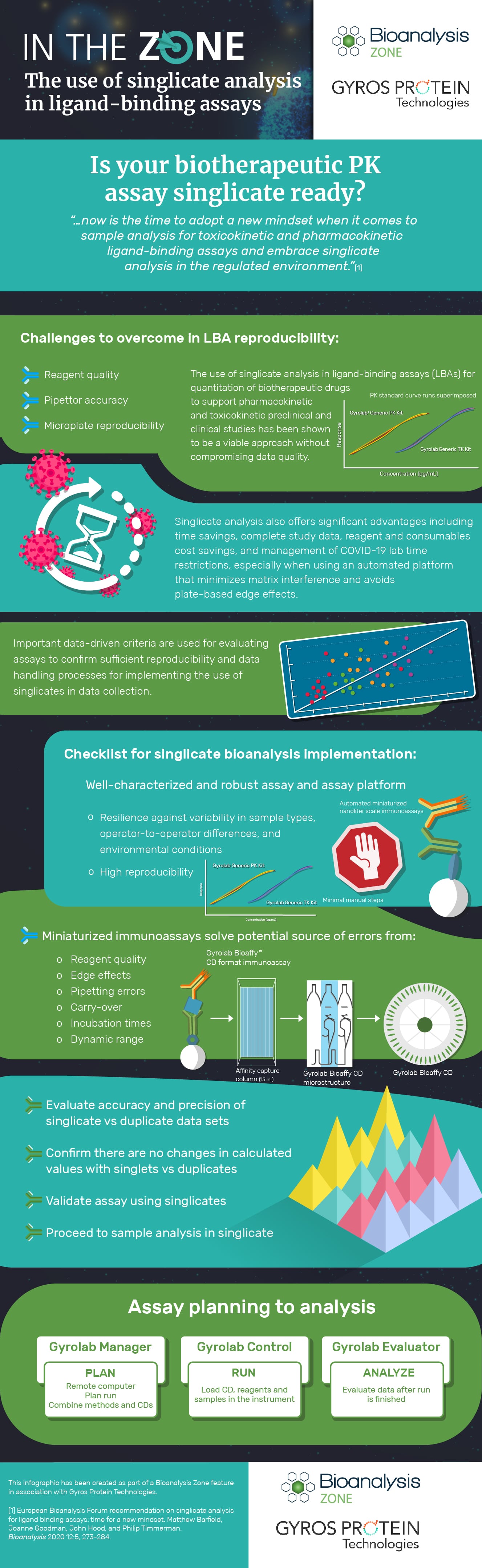



Is Your Biotherapeutic Pk Assay Singlicate Ready Bioanalysis Zone
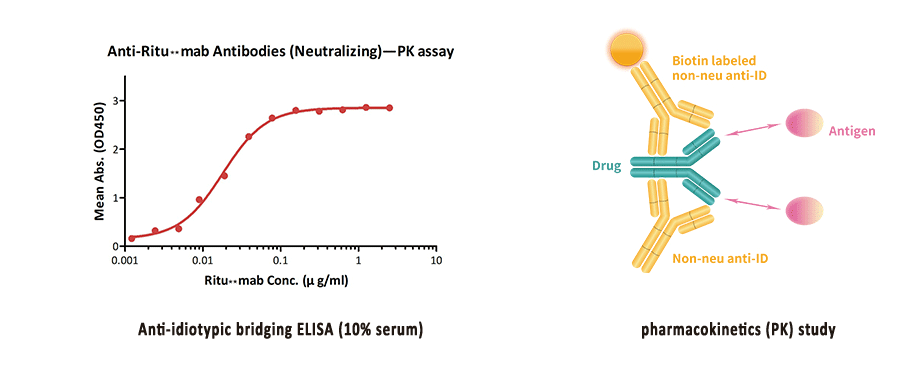



Innovation Lab Capabilities Acrobiosystems



Www Ldms Org Downloads Training Manual Windows Pharmacology Pdf Ver 5



Pk Biomarker Assessment Creative Biolabs
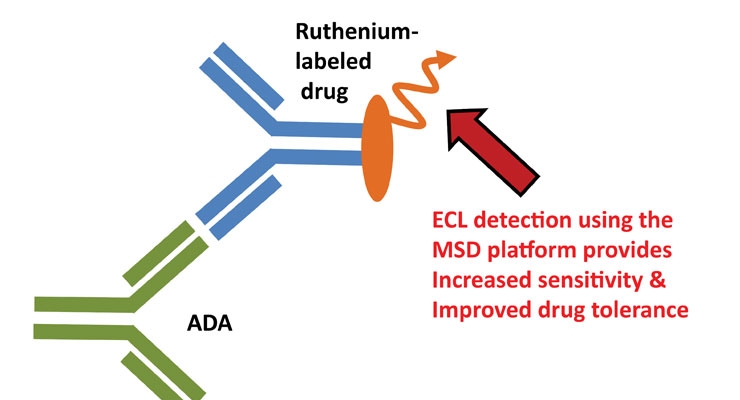



A Case For One Assay Analysis Of Pk And Immunogenicity In Biosimilar Research Contract Pharma




Frontage Develops A 3 In 1 Multiplex Pk Assay For Evaluation Of Hydroxy Chloroquine Chloroquine And Azithromycin In Human Plasma To Support Covid 19 Clinical Trials Frontage Laboratories



Pharmacokinetic Toxicokinetic Pk Tk Analysis Creative Biolabs



Elisa Protocols Bio Rad



1



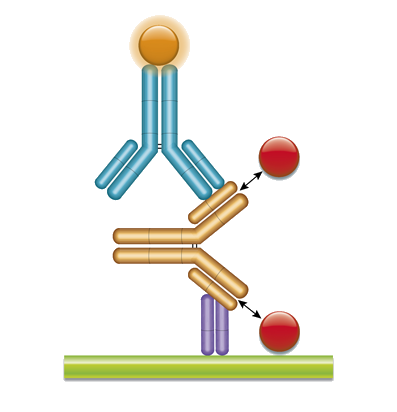
A Cell Based Assay For Evaluating Binding And Uptake Of An Antibody Using Hepatic Nonparenchymal Cells Scientific Reports




Ligand Binding Assay An Overview Sciencedirect Topics
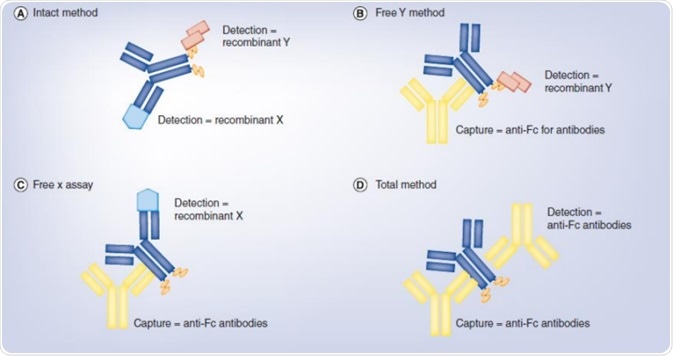



Identification Of A Bispecific Antibody With Bioactivity Analysis



Establish Drug Pk Analysis Method Based On Competitive Elisa




Readcoor How Can True Spatial Sequencing Be Applied To Accelerate Your Research Our Scheduled Webinars Sept 18 Cancer Immunotherapy Research Sept 23 Single Assay For Pk Pd Safety Profiling Oct




Specialty Lab Biomarker Assays Precision For Medicine




Pharmacokinetics At Kcas An Orchestrated Approach Kcas Bioanalytical Services




Adp Glo Kinase Assay Kit Promega Bioz Ratings For Life Science Research




A Robust And Versatile Nanobody Platform For Drug Delivery Biorxiv
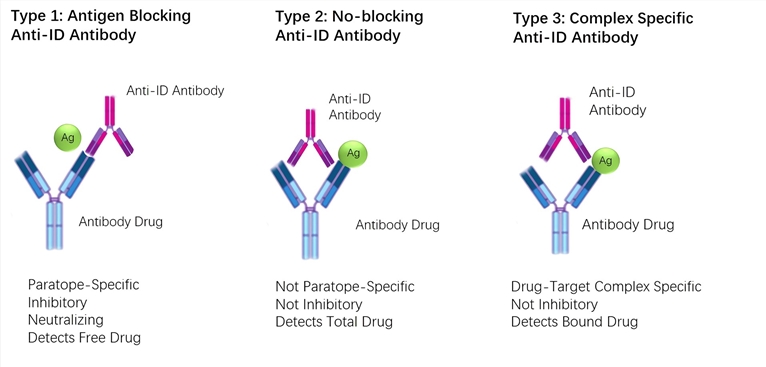



Pharmacokinetic Pk Bridging Elisa Measuring Free Drug Creative Biolabs
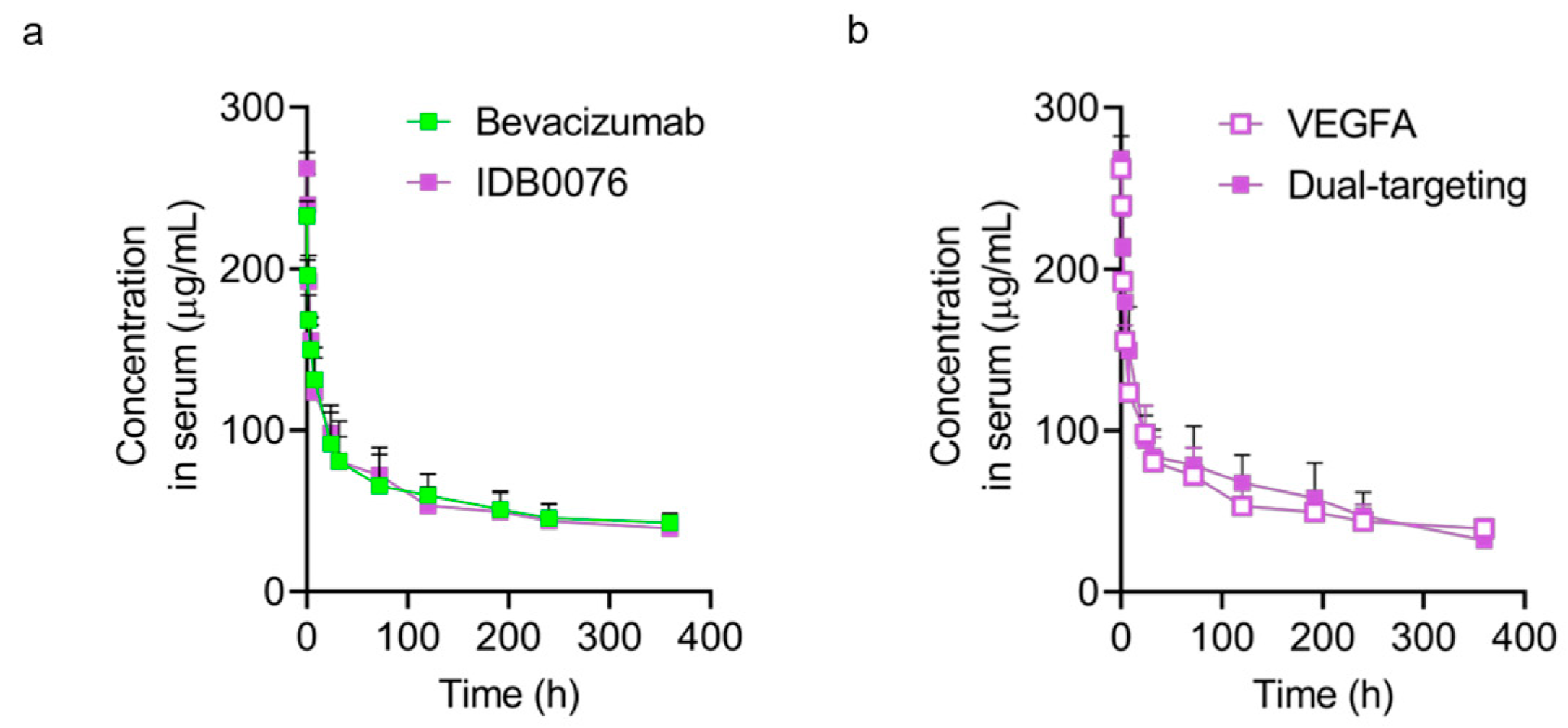



Biomolecules Free Full Text Preclinical Efficacy And Safety Of An Anti Human Vegfa And Anti Human Nrp1 Dual Targeting Bispecific Antibody Idb0076 Html




Pk Assay Development For Biologics Using Elisa Msd Immunoassay Platforms




Ultimate Sensitivity Pk Assay For Alibion Smv3 Ch




Pk Assay Analysis Services Pharmacokinetics Pk Study Pk Testing Northeast Biolab
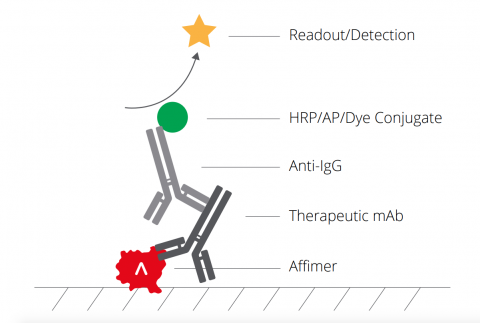



Improving Drug Development Processes With Antibody Pk Assays Avacta Life Sciences Limited




Effective Tools For Drug Monitoring Assays Bio Rad
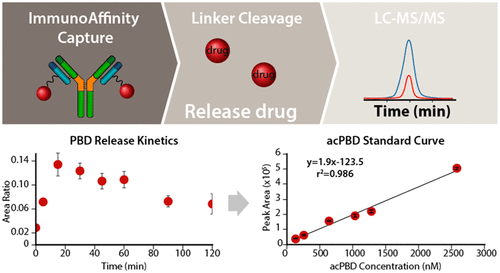



Conjugation Site Influences Antibody Conjugated Drug Pk Assays Case Studies For Disulfide Linked Self Immolating Next Generation Antibody Drug Conjugates Analytical Chemistry X Mol



Http Microbiology Ucdavis Edu Heyer Wordpress Wp Content Uploads 13 11 Atpase Assay Pdf




Theoretical Considerations And Practical Approaches To Address The Effect Of Anti Drug Antibody Ada On Quantification Of Biotherapeutics In Circulation Abstract Europe Pmc




Figure 3 From Quantification Of A Bifunctional Drug In The Presence Of An Immune Response A Ligand Binding Assay Specific For Active Drug Semantic Scholar



Resources Perkinelmer Com Lab Solutions Resources Docs App Alphalisa Pharmacokinetic Tnfa Pdf



Www Ldms Org Downloads Training Manual Windows Pharmacology Pdf Ver 5




Figure 3 From Development Of Pharmacokinetic Pk Assays For Detecting Biosimilars Targeting Tnfa Using Alphalisa Semantic Scholar
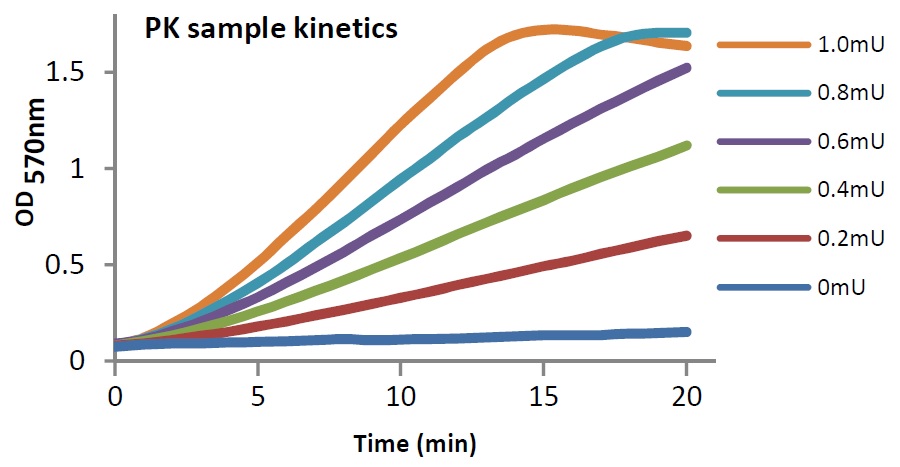



Pyruvate Kinase Pk Activity Kit Colorimetric Fluorometric Ls K642




Indirect Assessment Of Neutralizing Anti Drug Antibodies Utilizing Pharmacokinetic Assay Data Sciencedirect
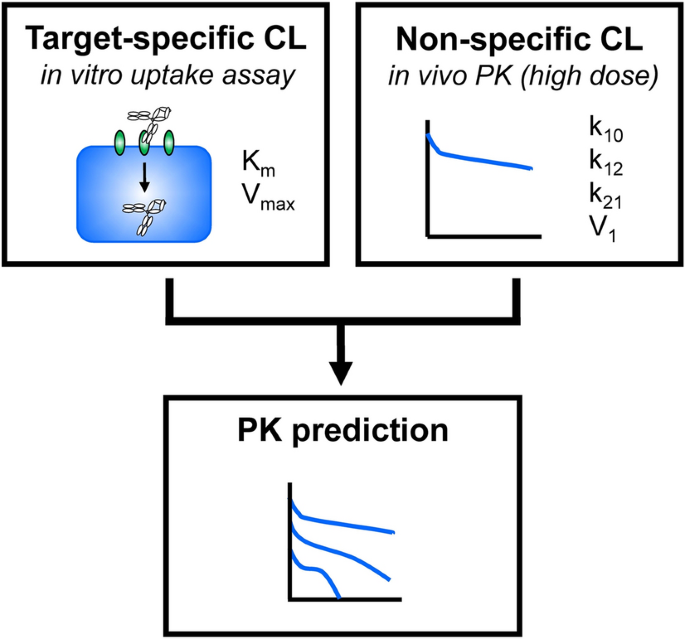



Pharmacokinetic Prediction Of An Antibody In Mice Based On An In Vitro Cell Based Approach Using Target Receptor Expressing Cells Scientific Reports



A Bispecific Antibody Based Assay Shows Potential For Detecting Tuberculosis In Resource Constrained Laboratory Settings
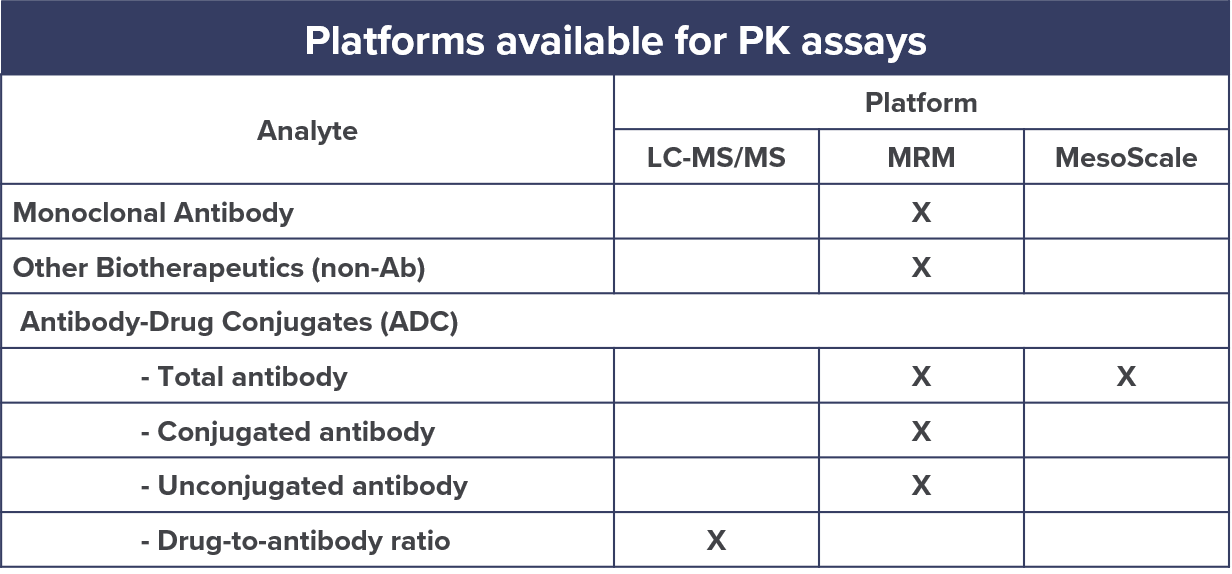



Pharmacokinetic Assays For Biologics Cellcarta




Pyruvate Kinase Activity Colorimetric Fluorometric Assay Kit K709 100 From Biovision Biocompare Com




Pharmacokinetic Pk Assays Pk Pd Analysis Dmpk Assay




Design Of Biosimilar Bioanalytical Programs Pk Assay Considerations




Ligand Binding Assays Certara




Anti Ox40l Clinical Pk Assay Developed On A Msd Ecla Schema Of The Download Scientific Diagram



Www Bostonsociety Org Images Apa 17 Biomarker Short Course Materials V2 Pdf




Automating And Miniaturizing Immunoassays




Qps Biosimilar Bioanalytical Approaches



Pk And Ada Assay Development Services




Overview Of Elisa Thermo Fisher Scientific Pk
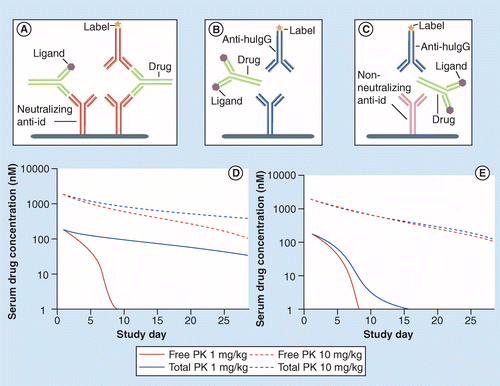



Pk Pd Modeling Of Protein Drugs Implications In Assay Development Bioanalysis




What Is An Anti Idiotypic Antibody




Mechanistic Projection Of First In Human Dose For Bispecific Immunomodulatory P Cadherin Lp Dart An Integrated Pk Pd Modeling Approach Chen 16 Clinical Pharmacology Amp Therapeutics Wiley Online Library




Pk Bundle



2



1




Ada Assay Formats Used For Cynomolgus Monkeys A Download Scientific Diagram



Q Tbn And9gcsw7npyr6dolfhphe1nrpexwsvxavlae Axibq4yiix14n Hnpj Usqp Cau




How To Improve Your Antibody Drug Development Assays With Anti Idiotypic Antibodies Youtube



Receptor Dimerization Assays




Use Of Different Secondary Antibodies In A Type 3 Pk Assay Human Erbb2 Download Scientific Diagram




Pyruvate Kinase Assay Kit Ab432 Abcam



Q Tbn And9gcsw7npyr6dolfhphe1nrpexwsvxavlae Axibq4yiix14n Hnpj Usqp Cau




B Arrestin Recruitment Assays




Covance Validate Affimer Reagents As Critical Pk Assay Reagents Avacta Life Sciences Limited




Pk Pd Chimera Biotec Gmbh



0 件のコメント:
コメントを投稿